

Ammosamides
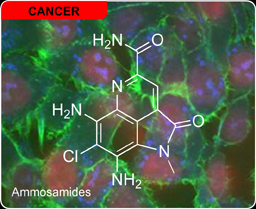
We have completed studies on a new family of pyrroloquinolines, the ammosamides A and B, in collaboration with the Fenical laboratory at the Scripps Insitution of Oceanography (structures published here). Using an immunofluorescence affinity (IAF) strategy, Chambers Hughes in the Fenical laboratory prepared an analog of ammosamide B that was used for both cellular microscopy and protein target indentification studies at XRI to identify myosin as a target of the ammosamides (read it here). The synthetic program was conducted at Scripps and the biological studies at our laboratory on Adams Avenue in San Diego. This work was subsequently highlighted in Nature Chemical Biology, spolighted in ACS Chemical Biology and highlighted in Angewandte Chemie (read it here).
Since its discovery, a series of total synthetiec fforts have been described including routes developed by Hughes and Fenical (read it here) and Cushman (read it here or here). Most recently, the MacMillian laboratory discovered a ring opened analog ammosamide D (read it here).
Copyright 2004-2012 | The Xenobe Research Institute | a California-based 501(c)(3) organization.