

Sceptrin
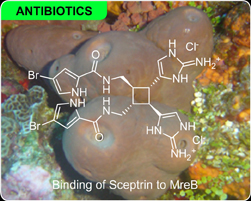
A bidirectional affinity system was used to screen for marine natural products that bound to Escherichia coli proteins. A system was developed and applied to isolate the natural product sceptrin from an Agelas conifera extract and its affinity partner MreB from E. coli lysate. The use of a dual immunoaffinity fluorescent (IAF) tag permitted this process to co-immunoprecipitate the bacterial equivalent of actin, MreB, from E. coli lysate. MreB was subsequently validated as a target for sceptrin using a resistance mapping approach. The combination of these studies suggests that natural products and their protein targets can be isolated in concert using a melody of forward and reverse affinity matrices. While the structure of sceptrin was elucidated by NMR analysis, the bulk of effort was conducted without knowing the structure of the natural product, thereby elevating a key bottleneck in the development of high-throughput methods for natural product discovery. This work recently appeared in the Journal of the American Chemical Society (read it now). These studies were conducted at our Adams Avenue facility in San Diego.
In 2010, a team lead by Baran and Vuori described the mechanisms in which sceptrin inhibits tumor cell motility (read it here). These studies identified a clear role for the actin cytoskeleton by sceptrin, and therein provided further support for the targeting of MreB in bacteria.
Copyright 2004-2012 | The Xenobe Research Institute | a California-based 501(c)(3) organization.