

Hexacyclinol
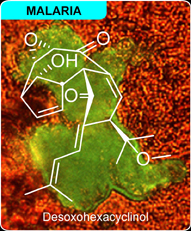
Desoxohexacyclinol was discovered in late 1999 from cultures of Panus rudis. This terpene was identified after screening ascomycete cultures using an proteomic affinity-based extraction method. Using this protocol, we identified BB302 from resins displaying proteins from Plasmodium berghei. Probe BB-302 displayed potent growth inhibition of P. berghei targeting a unique protein found on the oocyte cyst. Based on this evidence, a complete study of these cultures was conducted leading to the isolation and chacterization of desoxohexacyclinol and two related endoperoxides, hexayclinol and 5-epi-hexacyclinol.
In 2006, Dr. La Clair published the synthesis of desoxohexayclinol and its corresponding endoperoxides, hexacyclinol and 5-epi-hexacyclinol (read it now). This work was a culimination of efforts led by Dr. La Clair from 1999-2002. Through synthesis, this study delivered an endoperoxide that matched the data set of a compound isolated at the Hans-Knoll-Institute (HKI) in Jena Germany. At this point, structures isolated in our program were named based on the hexacyclinol motif. A few months after publication of this work, Rychnovsky published a structural revision of (+)-hexacyclinol based on the NMR data set published by HKI (read it now). This effort was followed by a second synthesis of (+)-hexacyclinol by Porco and Rychnovsky suggesting structural revision to an congener of panepophenanthrin (read it now).
There are a couple of puzzling questions arising from these efforts. First, recent concern was raised over the fact that the activities of the materials isolated by La Clair and that of panepophenanthrin, ubiquitin-proteasome inhibitor, and its congeners do not match. Our samples of the hexacyclinol endoperoxide delivered potent activity against P. berghei and subsequent mechanism of action studies identified covalent modification of a ferrochelase. Moreover, increasing evidence from 2D NMR methods has yieled clear NOE and coupling transitions that are different between the two motifs. Efforts are now underway to repeat the isolation of desoxohexacyclinol and the hexacyclinol endoperoxides and complete structural studies on it and its conversion to the hexacyclinol endoperoxide. One of the more important discoveries developed through our efforts has been largely unrecognized by the attention drawn to the structure elucidation issue. Namely, the conversion of a diene-precursor, desoxohexacyclinol, to endoperoxides via a 2+2+2 cycloaddition with oxygen. We believe that this process suggest a unique motif for in vivo synthesis of endoperoxides. Studies towards evaluating the use of this mechanistic hypothesis are ongoing..
In November of 2012, Dr. La Clair retracted his intiial studies (read it now) based on the fact that it lacked sufficient spectral data supporting the synthetic intermediates as well as detailed 2D NMR analyses on the isolated and synthetic final products; an omission that fueled considerable controversy. While many published synthetic and natural product isolation efforts can be found in the literature with similar lacks of data, the author elected to retract this paper due to the fact that this omission failed to meet the current expectations associated with the publication of total syntheses and natural product isolation. The author hopes that this incident inspires publishers, editors, reviewers and authors to advance more effective measures to check and require spectral data sets as a part of the publication process. In particular, there is currently a lack of tools for presenting raw data files along with publications.
Copyright 2004-2012 | The Xenobe Research Institute | a California-based 501(c)(3) organization.