

Cephalostatins - Ritterazines
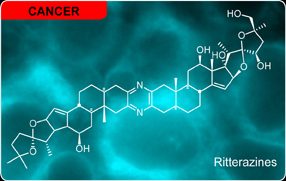
Ritterazine B, bis-steroidal pyrazine, extracted from Ritterella tokioka displays potent toxicity against a number of tumor cell lines. (read more here). The ritterazines, along with their structurally-related cephalostatins, comprise a family of structurally related natural products reported by Professors G. R. Pettit and N. Fusetani. Bryan R. Moser has recenly written an excellent review on this family (read it here). We have begun studies on determining the mode of action of the ritterazines in collaboration with the Fuchs laboratory (Purdue University). Here, the synthetic methods developed in the Fuchs laboratory are used to prepare milligram quantities of suitably active IAF ritterazine-cephalostatin hybrid (a so called ritterostatin) for cellular microscopy and target elucidation studies. This work conducted at our Adams Avenue facility was published in Organic Letters (read it here) We have now concluded detailed imaging studies in tumor cells and tissues and are now screening cells and cell lysates for protein or oligonucleotide targets. In 2011, a team led by the Shair laboratory describe the role of these natural products in regulating oxysterol-binding proteins (read it here).
Copyright 2004-2012 | The Xenobe Research Institute | a California-based 501(c)(3) organization.