

Phorboxazoles
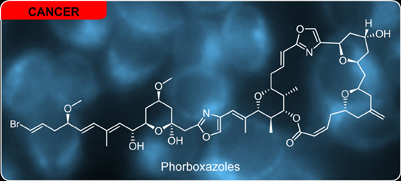
In 1995, Searle and Molinski reported the isolation and heroic structure elucidation of phorboxazole A and B from the marine sponge Phorbas sp. (read it now) Subsequent isolation efforts by Capon validated that observation (read it now). Over a decade after its isolation, synthetic efforts from the laboratories of Burke (read it now), Evans (read it now), Forsyth (read it now), Smith (read it now), Pattenden (read it now), Panek (read it now), Rychnovsky (read it now), Williams (read it now), and White (read it now) have provided a myriad of routes to install the novel features of the macrolide core and cyclic-hemiacetal bearing tail. The scale and scope of this work is truly profound.
Our efforts have focused on evaluating their mode of action. As supplies of the natural material are no longer available, these studies provide an ideal venue to display the advance of synthetic methodology. In collaboration with the Forsyth laboratory at Minnesota (now at Ohio State), we evaluated a panel of surroGATE labeled phorboxazole analogs. Through these studies, we determined that phorboxazole induced a unique interaction between cytokeratin and cdk4 (read it now). This event led to sequester of cdk4 onto filaments thereby preventing its translational delivery to the nucleus, and delivering arrest to cell. While recently supported, this mechanism offers new insight into anti-proliferative design.
Recent efforts with Bo Wang in the Forsyth group we have completed tissue and whole organism uptake studies. Here advanced intermediates prepared by Bo were used to evaluate the histological specification with regards to cell and tissue type. We have also recently determined that phorboxazoles induces more than one phenotype of cell death with a complex set of interactions being regulated by concentration, timing of exposure, and cell type. Moreover, further studies are needed to determine as to what role the association of natural products on keratins as well as other cytoskeletal assemblies has on “reprogramming” protein networks.
Copyright 2004-2012 | The Xenobe Research Institute | a California-based 501(c)(3) organization.